Introduction
Patients (pts) older than 60 years of age diagnosed with classic Hodgkin lymphoma (cHL) have worse prognosis than younger pts, including complete remission rates (CR) as well as lower progression free (PFS) and overall survival (OS). Age over 70 years, along with comorbidities, has been identified as a factor conferring additional worse prognosis. We sought to evaluate the prescription patterns and outcomes of pts ≥70y in comparison with “younger” elderly cHL pts diagnosed 60-69y.
Methods
We conducted a retrospective review of newly diagnosed cHL patients treated at our academic medical center between 2012-2022. Baseline and treatment characteristics, including planned and unplanned dose reduction events were collected from electronic medical records. Outcomes included disease response to initial therapy, PFS, OS and non - relapse mortality (NRM). All analyses were done using R and its packages. Differences between age groups were evaluated using Fisher and Wilcoxon rank-sum tests. PFS and OS were calculated using the Kaplan Meier method, comparisons done using log-rank. The cumulative incidence of NRM was calculated considering relapse as a competing risk, comparisons evaluated with Gray's test.
Results
We identified 81 pts ≥60 years (y) diagnosed with cHL, 37 of whom were ≥70y. Among baseline characteristics, more pts ≥70y had ECOG performance status ≥2 (30 vs. 9%, p = 0.05), and all had at least one comorbidity, compared with 77% in pts 60-69y (p = 0.002); other baseline characteristics were comparable, and the numerical difference in the rates of individual comorbidities did not reach statistical significance (table 1).
A statistically significant higher proportion of pts ≥70y had planned de-intensification of their treatment (46% vs. 16%, p = 0.007), most commonly this corresponded by prescription of the doxorubicin, vinblastine and dacarbazine (AVD) as initial therapy, use of brentuximab vedotin alone or in combination with non-anthracycline containing regimens. One pt in the younger cohort did not receive therapy and died within two weeks of diagnosis. Subsequent dose reductions occurred in both age groups and pts ≥70y had a higher proportion of pts with overall reduced treatment intensity (i.e., planned + unplanned) (n = 22, 59% vs. n = 14, 33%, p = 0.024).
Among pts treated with systemic therapy (n = 80), overall response rates were 73% and 85% for ≥70y and younger pts, respectively (p = 0.42). Complete response (CR) rates were 70% and 81% (p = 0.23). For pts treated with an anthracycline containing regimen, CR rates were 78% for pts ≥70y and 83% for pts 60 - 69y (p = 0.77).
After a median follow up of 53 months (IQR 32-71), 18 (23%) pts had progression of their disease after initial therapy and 18 (23%) pts had died. Median progression free survival had not been reached for both groups, and four-year estimates were 65% for pts ≥70y (95% CI 51-85%) and 83% for pts 60 - 69y (95% CI 73-95%), this difference did not reach statistical significance (p = 0.08). Similar results were observed for PFS when the analysis was limited to pts treated with anthracycline-containing regimens (4-year PFS: ≥70y = 68% vs. 60-69y = 85%, p = 0.06). Median OS was not reached for both groups, four-year estimates were 75% for pts ≥70y (95% CI 61-92%) and 90% for pts 60-69y (95% CI 81-100%) (p =0.007), with comparable results when pts treated with anthracycline were studied (4-year OS: ≥70y = 75% vs. 60-69y = 92%, p = 0.003).
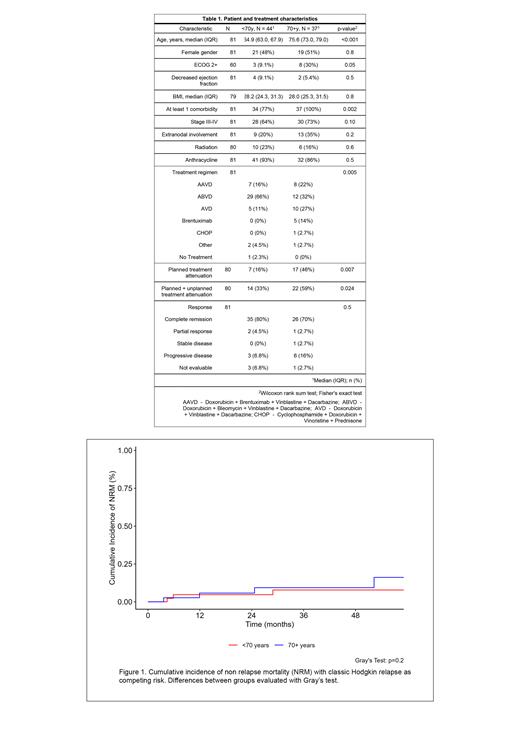
Analysis of the cumulative incidence of NRM (with relapse as competing risk) showed there were no significant differences among pts ≥70y (1-year 5.8% [95% CI 1-17%]) vs. 60-69y (4.7% [95% CI 1-14%]) (p = 0.2) (figure 1).
Conclusions
Patients diagnosed with cHL at ages ≥70 years have more comorbidities and more often have worse performance status than pts 60-69y. Older pts more often are treated with attenuated regimens and/or require dose reductions/discontinuation after starting therapy. While the dose reductions and modifications limit NRM to rates comparable to those of younger pts, PFS and OS remain lower for pts ≥70y, suggesting long - term disease control is inferior in this patient subgroup. Systematic prospective studies of attenuated but more effective regimens are needed in cHL pts diagnosed at advanced age.
Disclosures
Winter:Janssen: Consultancy; AstraZeneca: Consultancy; ADC Therapeutics: Consultancy; Seattle Genetics: Consultancy; BeiGene: Consultancy. Jagadeesh:Regeneron Pharmaceuticals: Research Funding; MEI Pharma: Research Funding; Trillium Pharmaceuticals: Research Funding; Affimed: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; LOXO Pharmaceuticals: Research Funding; Seagen: Research Funding; ATARA Biotherapeutics: Research Funding; Debio Pharma: Research Funding; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees. Hill:Gilead: Other: Advisory board; Incyte: Consultancy; Pharmacyclics: Consultancy, Other: Advisory board, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Genentech: Consultancy, Other: Advisory board, Research Funding; Bristol Myers Squibb: Consultancy; BeiGene: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy, Other: Advisory board, Research Funding. Caimi:ADC Therapeutics: Consultancy; SOBI: Honoraria; Lilly Oncology: Consultancy; BMS: Consultancy; Genentech: Consultancy; Novartis: Consultancy; Kite Pharma: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal